Major Developments in Pulmonary Hypertension Affecting Prognosis
1. Approval of initial therapy with Letairis and Adcirca
The AMBITION Study has reinforced the importance of an aggressive early regimen for the treatment of PAH. Although this study did not prove a mortality benefit, there were dramatic improvements in clinical worsening.
2. Availability of oral prostanoid medications (Orenitram and Uptravi)
Orenitram’s approval though an exciting development does not translate in measured improvements in outcomes for most patients studied. We should be cautious with this agent. In contrast, Uptravi was shown to reduce rates of clinical worsening in PAH patients.
3. New data published about the role of anticoagulation
Two recent publications attempted to shed light on the role of anticoagulation in PAH. Unfortunately, the message was very confusing for patients with idiopathic PAH. For patients with other types of PAH, the message was more consistent—no advantage to anticoagulation.
The Best Data Available to Determine Life Expectancy in Pulmonary Hypertension
We first need to acknowledge that we do not have a good way of predicting any one patient’s life expectancy. What we do have are data that look at several large groups of patients over many years. These data were collected for the most part in the early 2000’s as part of the Reveal Registry and thus do not reflect today’s best care. What this data showed us is that in this group of 2,635 patients, overall one year survival was 85%, three year survival was 68%, five year survival was 57% and seven year survival was 49%.
A closer look at this data also showed us that certain characteristics predict a greater likelihood of doing poorly. These characteristics are lumped together under the term “high risk features”. Certain characteristics predict a much better course and are termed “low risk features”.
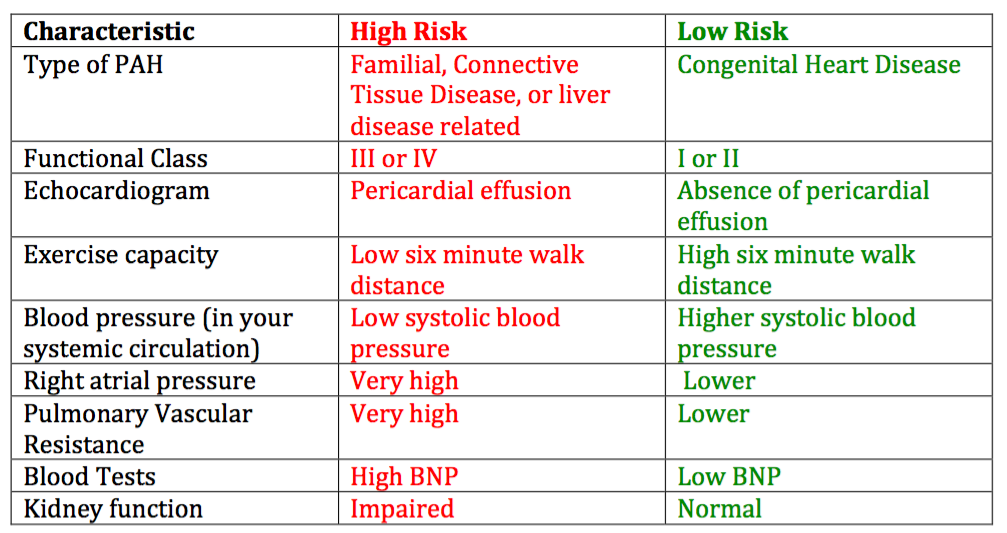
How Long Can I Expect to Live with Pulmonary Hypertension?
Although pulmonary arterial hypertension was discovered in 1891, there were no known treatments for the disease until 1994 when Flolan was introduced. Prior to the release of that medication, the prognosis and life expectancy for a patient with pulmonary hypertension was around 3 years and it took an average of 2 years to accurately diagnose a patient. Flolan was the first drug to increase the life expectancy by up to 5 years for pulmonary hypertension patients. While we do not currently have a cure for the disease, several more treatments have been approved for PAH thanks to research. Over the past 20 years, we have gone from no medications to treat PAH to over 10 medications. Even more medications are being studied. We are optimistic that our patients will continue to experience longer survival and better quality of life.
To reiterate, no doctor has a crystal ball. However, in general, patients that fit into the low risk group (above) live more than 10 years. As patients begin to acquire more and more high risk features, survival declines. In my patients that have all of the high risk features, despite my most aggressive combination of medications, we often begin talking about lung transplantation.
Researching Treatments and a Possible Cure for Pulmonary Hypertension
The best news of all for patients with PAH is that we have a rich pipeline of new medications being studied for PAH. We remain very optimistic that we will continue to improve our patient’s survival. I encourage my patients to be part of the process of improving patient outcomes by participating in clinical research trials.
Key players in Research for Pulmonary Hypertension
The advancements in treating pulmonary hypertension patients would not have been possible without research. There are  several key players in bringing a drug to market or making it available for patients. The drug companies who set up the research study for the specific drug, the pulmonary hypertension centers that become participating sites for the studies, and most importantly the pulmonary hypertension patients who enroll in the research studies. Research is such an important piece of bringing new therapies to market that the PHA (Pulmonary Hypertension Association) requires all of their Comprehensive Care Centers to have a fully functioning research department.
several key players in bringing a drug to market or making it available for patients. The drug companies who set up the research study for the specific drug, the pulmonary hypertension centers that become participating sites for the studies, and most importantly the pulmonary hypertension patients who enroll in the research studies. Research is such an important piece of bringing new therapies to market that the PHA (Pulmonary Hypertension Association) requires all of their Comprehensive Care Centers to have a fully functioning research department.
Who can Participate in Pulmonary Hypertension Research
Each research study has strict rules about what types of patients can be enrolled, how the drug will be administered, and what measurements will be used to determine the results of the study. Many of the studies have requirements of what background therapy a patient can be on before they are enrolled in the study. For example, protocol (rules of the study) might say a patient cannot be on any treatments for pulmonary hypertension prior to enrolling in the study or protocol might say they can be on Adcirca or Revatio but not Letairis or Tracleer. It is becoming more and more difficult to find pulmonary arterial hypertension patients to enroll in studies as many of them are starting therapies with physicians outside of specialty clinics and disqualifying themselves as research candidates.
Steps to Participating in Research for Pulmonary Hypertension
 Most pulmonary hypertension centers of excellence participate in research studies. If the physician you are currently working with does not participate, inform them that you would like to learn more about research studies for treatments for pulmonary hypertension. They may be able to refer you to a center currently enrolling patients in studies.
Most pulmonary hypertension centers of excellence participate in research studies. If the physician you are currently working with does not participate, inform them that you would like to learn more about research studies for treatments for pulmonary hypertension. They may be able to refer you to a center currently enrolling patients in studies.
Research is completely separate from clinical management of the patient, meaning your current physician will continue to participate in your care. It is important for potential research patients to understand the risks and benefits of enrolling in a research study and what will be expected of them throughout the study. The patient will be asked to take home information about the study and read through it and come back to discuss any questions or concerns. Once all of their questions are answered they will be asked to sign an informed consent if they are interested in participating. It is important to understand that while a physician may offer the patient the chance to participate in a study, the decision is always up to the patient. Some patients will want to participate in research but not fit the specific rules of the current research studies enrolling. The sponsor (usually a drug company) of the study is ultimately responsible for deciding which patients are eligible to enroll. Once you are enrolled in a research study, you may choose to stop at any time. This is called withdrawing consent. You will not be punished or suffer any negative consequences from your doctor if you choose to stop participating. Some studies last a few months and others last many years.
We are very excited about research. Through your participation we are able to improve the care of all patients with PAH.
