Uptravi was approved in 2016 to treat Pulmonary Arterial Hypertension, PAH. We now have more than four years of experience with this medication. There are three broad pathways that PAH medications impact.
3 Pathways PAH Medications Impact
The endothelin pathway is overactive in PAH and leads to excessive blood vessel squeezing and stiffness. Medications such as ambrisentan (Letairis), macitentan (Opsumit), and bosentan (Tracleer) block the endothelin receptor and improve pulmonary artery function.
The second pathway involved in PAH is the nitric oxide pathway. PAH patients don’t have enough nitric oxide in their blood. Nitric oxide leads to relaxing of the pulmonary arteries and reduces pulmonary artery stiffness. Medications such as sildenafil (Revatio), Tadalafil (Adcirca, Alyq) and riociguat (Adempas) improve pulmonary artery function by increasing signaling in the nitric oxide pathway.
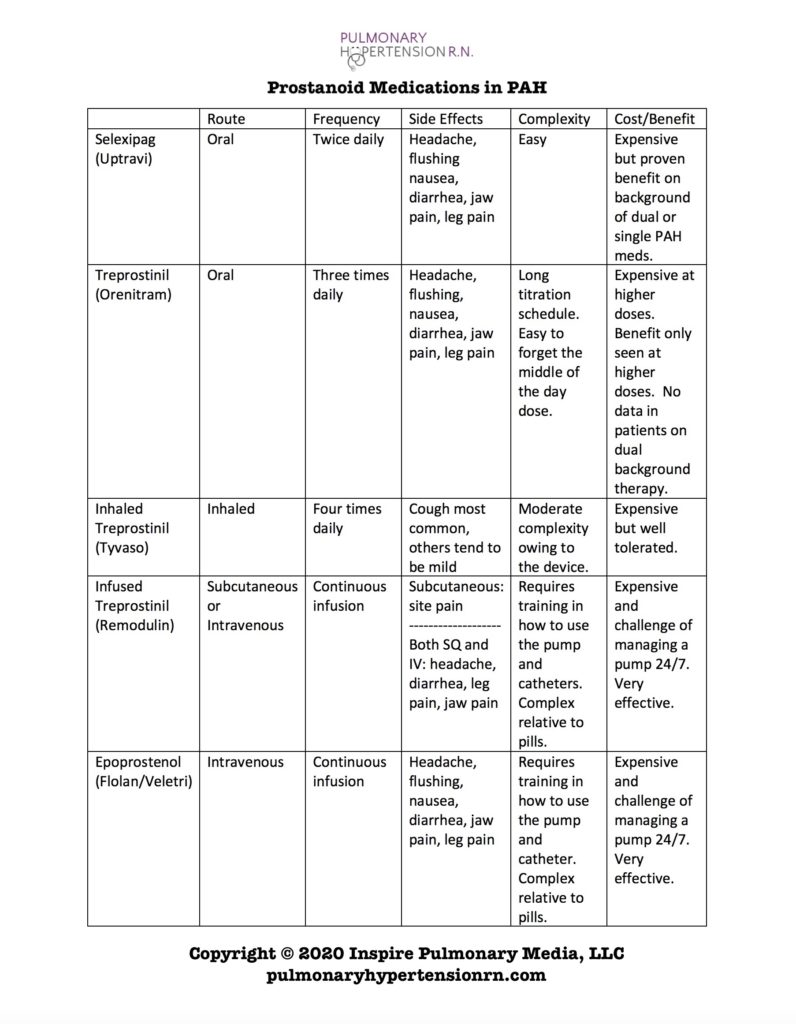
Lastly, PAH patients have reduced levels of prostacyclin. This hormone improves pulmonary artery function. Medications that act in this pathway are often referred to as prostanoids or prostaglandin receptor agonists. Such medications include Epoprostenol (Flolan or Veletri), treprostinil (Remodulin, Tyvaso, Orenitram) and selexipag (Uptravi). Only Orenitram and Uptravi are oral medications (pills).
Selexipag (Uptravi) Study
In December of 2015, the results of the GRIPHON study were reported in the New England Journal of Medicine. This was the largest PAH drug trial conducted, involving 1156 patients. The study was performed at 181 centers in 39 countries around the world. In the United States, the majority of patients were taking background approved PAH medications. Either selexipag (Uptravi) or placebo was added to their regimen. Starting at 200mcg of selexipag twice daily, the dose of either selexipag or placebo was gradually increased until side effects prevented further increases. Patients were then followed until enough events (clinical worsening events such as hospitalization, need for lung transplantation, death) occurred. Taking selexipag compared to placebo lead to a 40% reduction in the likelihood of worsening. Most of the events of worsening were hospitalization.
Like all prostanoid medications, side effects were common. Patients taking selexipag experienced headache, nausea, diarrhea, jaw pain, leg pain and muscle aches much more often than the placebo patients. In general, side effects were manageable with supportive medications addressing pain, nausea and diarrhea.
Where Does Selexipag Fit into Our Current Treatment Algorithm?
Due to the high cost, complexity and burden of side effects, most experts use selexipag as the third medication to treat PAH. There is broad consensus that for many patients, the combination of an endothelin receptor antagonist (ERA) such as Ambrisentan or macitentan and tadalafil or sildenafil should be first line. Patients that still need additional therapy are candidates for selexipag. Patients not meeting treatment goals despite dual oral therapy with and ERA and tadalafil are also candidates for oral and inhaled treprostinil (Orenitram or Tyvaso) in addition to infused prostanoid medications such as treprostinil or epoprostenol (Remodulin or Flolan/Veletri).
Although all treatment decisions require a detailed discussion between physician and patient, it is my general practice to prefer continuously infused treprostinil (Remodulin) over selexipag for patients that are very sick with PAH. For patients that need additional therapy but are not quite as ill, an oral or inhaled option is reasonable. Below you will find a side by side comparison for the prostanoid medications.